Combined Cycle Journal recently published an article on turbine lube oil varnish written by Peter Dufresne Jr. and his team at EPT. We’ve divided the article into four blogs to make it easier to digest -- click here to find parts one, two and four.
The Varnish Cycle
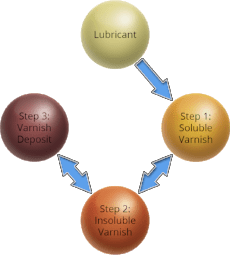
The typical varnish formation cycle in a gas turbine involves these three steps.
Step 1: Soluble Varnish
Oxidation is a chemical reaction between the lubricant base stock and oxygen present in the air surrounding it. Oxidation is unavoidable and begins to take place the instant that a new fluid is exposed to air for the first time, regardless of whether or not the fluid is put into service.
Like all other chemical reactions, the rate of oxidation is bound by the Arrhenius equation, which states that the rate of reaction will double for every 18°F (10°C) increase in temperature. Once a new fluid is put in service, it is exposed to higher temperatures and experiences a concomitant increase in the rate at which it oxidizes.
Even when operating temperatures are a typical 125°F (52°C), bearings may reach temperatures in excess of 300°F (149°C); the rate of oxidation at the bearing in this instance will be more than 1000 times greater than that in the cooler regions of the system. As a result, oxidation typically occurs wherever hot spots are found.
Oxidation products build up in the lubricant over time, but remain dissolved at operating temperatures unless they exceed the fluid’s saturation point.
Step 2: Insoluble Varnish
As the oil moves from hotter regions within the system to cooler ones (hydraulic lines supplying high-pressure oil to engine geometry control, for instance), the fluid temperature falls and the solubility of any varnish precursors present decreases.
These precursors begin to precipitate from solution in the form of particulates. Like water freezing to form ice, this precipitation of varnish is a physical change and not a chemical reaction.
Step 3: Varnish Deposits
Once formed, varnish particles agglomerate and form deposits, preferentially coating metal surfaces. These deposits are often the cause of unit trips or fail-to-start conditions. Like precipitation in Step 2 above, agglomeration and deposition are physical changes.
This model of varnish formation is widely accepted and reasonably well understood. Less well understood is the fact that once varnish deposits form, they can be reabsorbed, if the solvency of the lubricant is increased.
While the chemical changes that lead to the formation of varnish precursors (Step 1) are irreversible, the physical changes (Steps 2 and 3) which lead to the formation of varnish deposits are reversible. Successful varnish mitigation strategies use this fact to their advantage.
Testing for Varnish
As a result of the potential for costly turbine downtime associated with varnishing, it is imperative that a lubricant’s propensity to form varnish deposits be determined. Most turbine users test their lubricants for varnish potential using widely adopted techniques including QSA® (quantitative spectrophotometric analysis) and the standardized test MPC (membrane patch colorimetry, ASTM 7843). Proprietary (non-standardized) varnish test methods are not recommended, as they are not widely used and cannot be readily corroborated. Other collaborative analyses, like patch weight, may be helpful in substantiating oil health.
Both of the above varnish measurement methods can produce results which vary significantly depending upon the length of time during which the oil sample was “aged.” Indeed, longer sample aging periods produce higher MPC values, suggesting that degradation of lubricants continues in the sample bottle. For this reason, the ASTM MPC method suggests all samples be incubated at room temperature for 72 hours after being heated to 140°F (60°C) for 24 hours. This well-defined and standardized aging time has provided inter-laboratory consistency and improved repeatability.
Increasing MPC and QSA values during sample aging occur as a result of the continuing propagation of oxidation reactions that were likely initiated when the lubricant was in service. Oils that continue to degrade in a sample bottle will also continue to degrade in a lubricant reservoir. This highlights the necessity of using varnish removal equipment on a continuous basis. In the absence of varnish removal equipment, lube oil reservoirs with accumulations of dissolved breakdown products can continue to form varnish when the turbine is not operating.
You're 3/4 of the way to being a varnish expert now. Head to the final post in the series, where we tie it all together with varnish removal strategies.
Dufresne Jr., Peter "Lubricant Varnishing and Mitigation Strategies." Combined Cycle Journal. Q4 2013: Pages 34-40.
Print.







